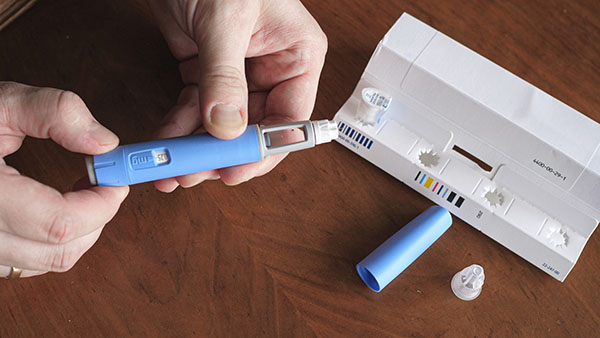
Torrent Pharmaceuticals withdrew the popular hypertension drug losartan last year after finding that the medication contained an unacceptably high level of a known carcinogen. The drug is commonly used to treat people with high blood pressure as well as diabetes patients with nephropathy.
The firm originally withdrew a few batches of the drug after the impurity was revealed, but they later expanded that recall from 2 to 10 lots and have since announced several additional expansion recalls. The fifth expansion recall for losartan potassium tablets also included losartan potassium hydrochlorothiazide tablets because they were found to contain nitrosodiethylamine, or NDEA.
These tablets were sold across the nation at retailers such as Walmart. This contaminant behind the recall is the same one that lead Novartis to stop distributing a generic version of the heartburn drug Zantac.
The recalls of losartan came among a wider string of recalls of blood pressure medications such as irbesartan and valsartan. The FDA said that the contaminants, which also included NDMA and NMBA, were likely the result of a manufacturing change adopted by the pharmaceutical factories in China and India that make the ingredients for the drugs. This change could create the contaminants in the presence of specific chemicals and reaction conditions and may be the result of reusing materials like solvents.
NDEA can cause liver damage and has been ”reasonably anticipated” to cause cancer in humans. Nitrosamines are formed when nitrates or nitrites react to secondary or tertiary amines. Although they are sometimes present in small amounts in foods such a smoked meats, certain vegetables and drinking water supplies, their presence in medications has been deemed a serious health concern because of their ability to cause cancer.
Meanwhile, NDMA is considered a probable human carcinogen because of its behavior in animals by both the Environmental Protection Agency and the International Agency for Research on Cancer. The FDA's maximum daily exposure level for NDMA is set at 96 nanograms, which is 600 times smaller than a grain of salt. Some of the cancers that have been linked to exposure to NDMA include those of the bladder, esophagus, stomach, pharynx, uterus, liver and colon.
Last year, the FDA admitted that some versions of valsartan had trace amounts of a carcinogen for four years before the impurity was detected by regulators, and there could well be countless other medications that have been contaminated that have not yet come to light.
These problems have prompted consumer advocate groups to call on the FDA to ramp up its efforts to ensure that medications are safe. At the very least, they should be inspecting all medications across all manufacturers a lot more thoroughly than they currently are.
It's all part of the Big Pharma racket
Predictably, the FDA is encouraging people not to stop taking their medications, warning them that suddenly stopping hypertension drugs can be risky.
This is all part of the Big Pharma racket. People who are concerned about their heart health are convinced to take high blood pressure medications by their doctors, and then they cause other side effects that make patients need even more drugs – or in this case, cancer, which means they could end up needing very profitable chemotherapy.
It’s all part of a nightmarish pattern that keeps repeating itself. If you take statins, you might develop a metabolism disorder. If you take diabetes drugs, watch out for liver disease. And no matter what you take, the drug companies win. Pharmaceuticals are a business, and it’s a very profitable model that you could pay for with your life.
Sources for this article include:
Please contact us for more information.