EMA's Pharmacovigilance Risk Assessment Committee (PRAC) initially examined data on people using liraglutide (Saxenda) and semaglutide medicines after being flagged by the Icelandic Medicines Agency about three cases of new complications – two cases where people had suicidal thoughts while using Ozempic and Saxenda and one case of self-harming thoughts while using Saxenda.
Liraglutide and semaglutide medicines are widely used, with exposure of over 20 million patients years to date. One patient-year is the equivalent of one patient taking a medicine for one year, EMA said in a statement.
It is not yet clear whether the reported cases are linked to the medicines themselves or to other factors like the patients' underlying conditions.
The review is being carried out in the context of a signal procedure – whether the drugs should note suicidal behavior as a side effect. A signal is information on a new adverse event that is potentially caused by a medicine or a new aspect of a known adverse event that warrants further investigation. The presence of a signal does not necessarily mean that a medicine caused the adverse event in question.
Saxenda and Wegovy are authorized for weight management in people who are obese or overweight in the presence of at least one weight-related health problem.
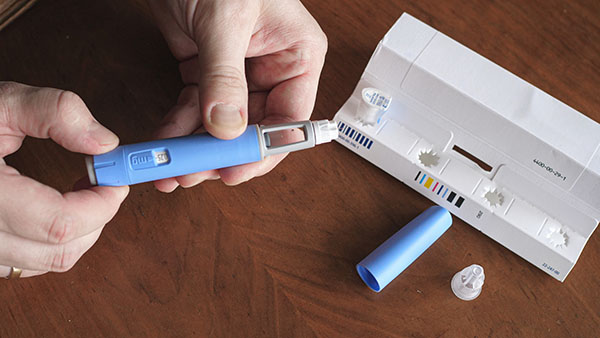
Ozempic is authorized for the treatment of adults with insufficiently controlled Type 2 diabetes, but has also been used off-label for weight loss.
Suicidal behavior is not currently listed as a side effect in the European Union product information for any GLP-1 receptor agonists. (Related: Experts warn: Popular weight-loss drugs could cause DEADLY side effects.)
The review of Ozempic, Saxenda and Wegovy started on July 3 and has now been extended to include other GLP-1 receptor agonists. This review is expected to conclude in November.
EMA will release results of the investigation when available. If one of the incriminated medicines is deemed dangerous, regulatory action like suspension of marketing authorization or withdrawal of the medicine from the market may be taken to ensure patient safety, EURACTIV.com reported.
Social media helps weight-loss drugs become popular
Social media posts about people shedding large amounts of weight have led to big demand for these types of treatment.
All medicines have potential side effects. For weight-loss drugs, side effects include constipation, diarrhea, headaches, nausea, stomach ache, tiredness and vomiting. Prescribers are also advised to monitor for any mental changes, especially sudden changes in the mood, behaviors, thoughts or feelings of patients.
Manufacturer Novo Nordisk is working with the EMA and says patient safety is a top priority.
"The safety data collected from large clinical-trial programs and post-marketing surveillance have not demonstrated a causal association between semaglutide or liraglutide and suicidal and self-harming thoughts,” Novo Nordisk said in a statement to CNBC on July 10.
The U.K.'s drug regulator, the Medicines and Healthcare products Regulatory Agency (MHRA), said it was monitoring the situation.
"As part of our close monitoring, any emerging evidence is routinely considered alongside other sources of information, including suspected adverse drug reactions. We will communicate any new advice to healthcare professionals and patients if appropriate," said MHRA Chief Security Officer Dr. Alison Cave.
Cave urged users to seek immediate medical assistance if they experience suicidal thoughts or thoughts of self-harm and asked everyone to report any suspected side effects using the MHRA Yellow Card scheme website.
Visit DangerousMedicine.com for more stories about the harmful side effects of pharmaceutical drugs.
Watch this video about the side effects of Ozempic.
This video is from the Dr. Jane Ruby channel on Brighteon.com.
More related stories:
Big Food, Big Pharma colluding to keep Americans DIABETC and OBESE to maximize profits.
With 1 in 5 kids now obese, Pharma sets sights on $50B market for weight-loss drugs.
Sources include:
Please contact us for more information.