- The FDA has placed a hold on trials for Moderna's mRNA-based RSV vaccines in infants due to severe side effects.
- Moderna's mRNA-1345 and mRNA-1365 vaccines were found to cause higher rates of serious respiratory infections in babies aged 5 to 8 months.
- Five severe RSV infections were observed in the mRNA-1345 group, compared to just one in the placebo group, leading to an immediate halt of trials.
- The FDA's caution highlights the potential for mRNA vaccines to exacerbate the conditions they aim to prevent, raising concerns about their safety.
- These developments could impact the future of mRNA vaccine development for other respiratory illnesses, emphasizing the need for rigorous safety evaluations.
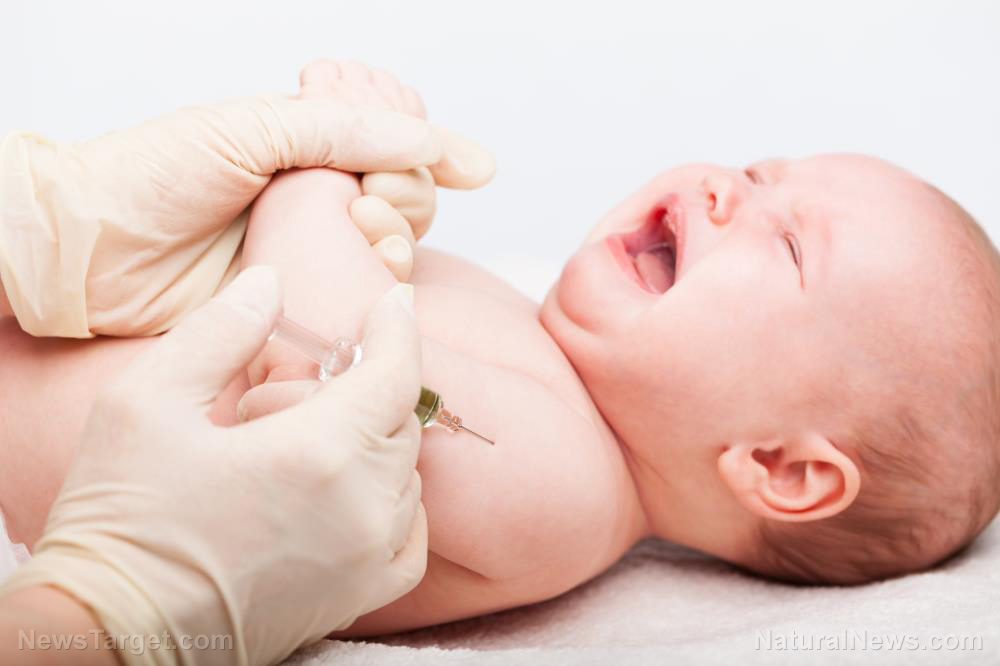
In a startling development, the U.S. Food and Drug Administration (FDA) has revealed that Moderna’s mRNA-based respiratory syncytial virus (RSV) vaccine candidates are associated with severe side effects, including higher rates of serious respiratory infections, in infants. The findings have prompted the FDA to put a hold on all investigational trials for RSV vaccines in babies and toddlers, raising serious concerns about the safety of these experimental vaccines.
Moderna’s mRNA-1345 and mRNA-1365 vaccines were designed to protect infants against RSV, a common respiratory virus that can lead to severe illness and hospitalization. However, clinical trials conducted on babies aged 5 months to 8 months revealed a concerning imbalance in severe or very severe respiratory syncytial virus lower respiratory tract infections (LRTI) among those who received the experimental vaccines.
In one study, five severe cases of RSV infections were observed in the group given the mRNA-1345 vaccine candidates, compared to just one case in the placebo group. This imbalance led to the immediate halt of Moderna’s pediatric RSV vaccine program on July 17, 2023, after the company was notified of the safety signal.
Further investigations revealed that these severe outcomes in babies were not limited to one vaccine candidate and were observed in both mRNA-1345 and mRNA-1365. The severity of these outcomes underscores the potential for mRNA vaccines to exacerbate rather than alleviate the very conditions they were designed to prevent—a phenomenon known as vaccine-associated enhanced respiratory disease (VAERD).
The FDA is not alone in its caution toward mRNA RSV vaccines. In a related development, Moderna’s mRNA-1345 vaccine, approved for adults aged 60 and older, is facing new scrutiny. Recent reports suggest that the vaccine may be exacerbating the very conditions it aims to prevent. This echoes a long-standing concern about mRNA vaccines, which, despite their promise, have shown a propensity to cause unintended immune responses that may be harmful, especially in vulnerable populations like the elderly and infants.
Moderna's mRNA vaccines for COVID-19 are also risky
Moderna’s track record with mRNA vaccines is particularly troubling. The company’s mRNA COVID-19 vaccines, widely distributed during the pandemic, have also been linked to serious adverse events, including the development of myocarditis and severe allergic reactions in some recipients. These incidents raise serious questions about the overall safety profile of mRNA vaccines and the adequacy of the testing and regulatory processes that allowed them to be deployed at scale.
The implications of these findings are far-reaching, potentially derailing the development of mRNA vaccines not only for RSV but also for other respiratory illnesses. The FDA’s decision to convene an advisory committee to discuss these safety concerns highlights the urgent need for a thorough reevaluation of the risks associated with mRNA vaccines, especially in infants and young children.
In light of these revelations, healthcare providers, parents, and public health authorities must carefully weigh the potential risks and benefits of mRNA vaccines, particularly for those most vulnerable to severe illness. The FDA’s actions serve as a critical warning that the rush to develop and roll out new vaccines must be balanced with rigorous safety evaluations to ensure the well-being of the populations these vaccines aim to protect. It's a lesson that many learned the hard way with COVID-19 vaccines, and the idea that babies could be in danger from yet another vaccine meant to protect them is deeply unsettling.
Sources for this article include:
Please contact us for more information.