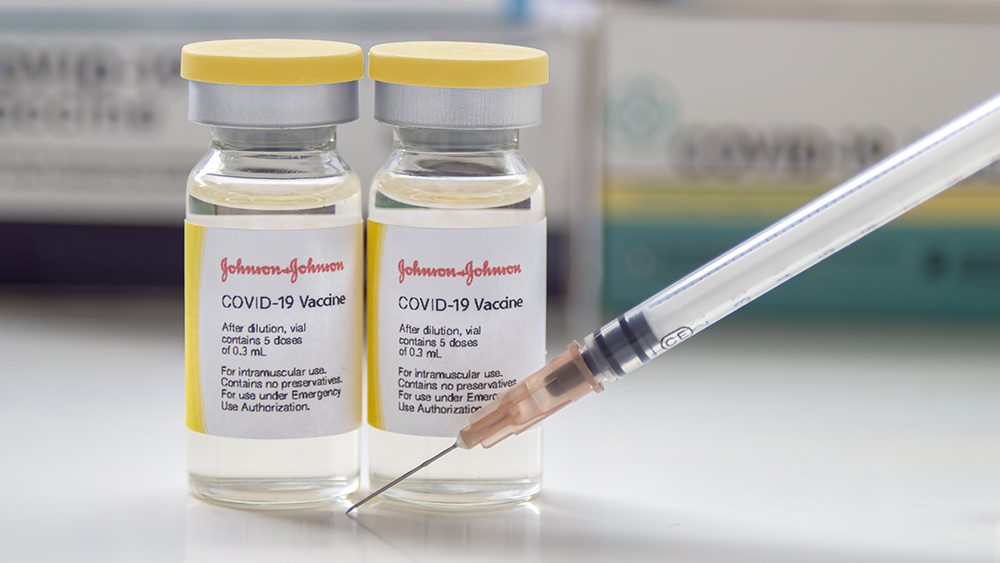
The incident occurred at Emergent Biosolutions, a factory in Baltimore, Maryland that Johnson & Johnson had contracted out to produce its vaccines. Johnson & Johnson said in a statement:
"This quality control process identified one batch of drug substance that did not meet quality standards at Emergent Biosolutions, a site not yet authorized to manufacture drug substance for our COVID-19 vaccine. This batch was never advanced to the filling and finishing stages of our manufacturing process."
"As with the manufacturing of any complex biologic medication or vaccine, the start-up for a new process includes test runs and quality checks to ensure manufacturing is validated and the end product meets our high-quality standards. This approach includes having dedicated specialists on the ground at the companies that are part of our global manufacturing network to support safety and quality."
The spoiled batch could have produced as many as 15 million doses of the Johnson & Johnson coronavirus vaccine. (Related: Yearly COVID-19 shots might be needed by everyone, Johnson & Johnson CEO now conveniently claims.)
The Emergent Biosolutions factory where the incident occurred has had other problems in the past, according to the Food and Drug Administration's (FDA) last inspection of the factory. Reports have previously come in about how the site's location had cracked vials and mold around one of its facilities.
Furthermore, the FDA had previously flagged another Emergent plant in Baltimore where the factory in question failed to test its ingredients for anthrax. This led the investigator in charge of the inspection to report that the company was failing to train its employees "in the particular operations they perform as part of their function and current good manufacturing practices."
The incident has also raised concerns about how many other Johnson & Johnson vaccines were erroneously made.
As of April 2, the Centers for Disease Control and Prevention's vaccine tracker shows that nearly 7.9 million Johnson & Johnson vaccines have been delivered.
The company is committed to manufacturing 80 million doses of its vaccine by the end of May, and 100 million doses by the end of June. Johnson & Johnson said it is also still on track to deliver more than one billion doses around the world by the end of the year.
FDA to conduct probe on what caused vaccine batch to fail quality control
The FDA has announced that it is opening an investigation to figure out what caused a batch of Johnson & Johnson's vaccines to go bad.
The agency said it may send an inspection team to assess the situation in Emergent Biosolutions' plant in Baltimore.
Emergent Biosolutions said in a statement on Thursday, April 1, that it was able to isolate the batch that did not meet Johnson & Johnson's quality standards and that it would dispose of the batch properly.
Meanwhile, Johnson & Johnson said that while discarding a whole batch of vaccines was disappointing, it could happen from time to time because of the "complex vaccine manufacturing process." The company also said the lapse in quality from this one batch has not affected the overall quality of the vaccine doses that have already been distributed in the United States.
Johnson & Johnson further claims to have enough supply of vaccine doses to meet its near-term commitments, despite the setback of losing 15 million vaccines.
The company said it was sending experts in technical operations, quality and manufacturing to the Emergent Biosolutions plant to oversee all manufacturing of the vaccine.
On Thursday, the White House responded to the incident with Johnson & Johnson by saying that it did not expect this setback to affect the company's promised supply of vaccines. The administration of President Joe Biden has already agreed to purchase 100 million doses of Johnson & Johnson's vaccine for $1 billion. The federal government expects these doses to be delivered by the middle of the year.
"We have been assured that they expect to meet those deadlines," said White House Press Secretary Jen Psaki.
Learn more about the many problems arising with all of the coronavirus vaccines by reading the latest articles at Vaccines.news.
Sources include:
Please contact us for more information.