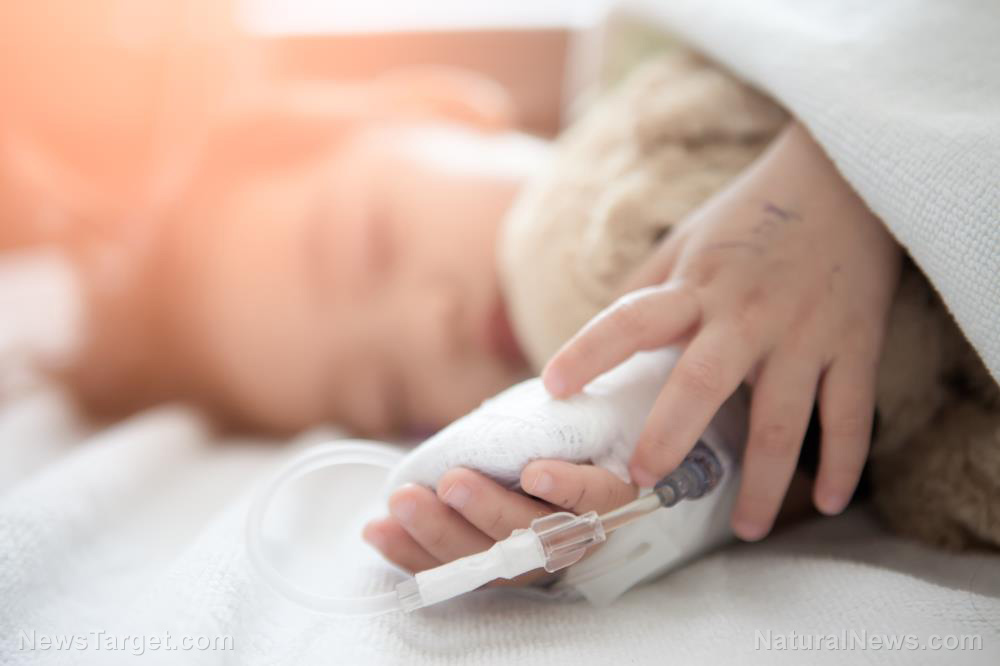
Researchers identified that the drug-resistant property of the El Tor strain of Vibrio cholerae (the bacteria primarily responsible for cholera) was because of the difference in how its enzyme responded to antibiotics, as opposed to other bacteria. In their study, which was published in the Journal of Biological Chemistry, they explained how the enzyme from the El Tor strain had a "different mechanism of action" than other proteins that have been studied in bacteria. The team believes that by learning how the process works, researchers will be able to find ways to address the increasing challenges of antibiotic resistance.
The El Tor strain, referred to by scientists as Vibrio cholerae O1 biotype El Tor, is the dominant strain for the seventh cholera pandemic, which began in 1961 in Indonesia and has since infected an estimate of three to five million people each year. The first instance of the strain was identified in the Middle East in 1897 as a nonpathogenic strain. However, it rapidly diversified into a virulent strain when it migrated to Makassar, Indonesia. Experts were able to point out the toxin changes in its genetic material in 1954, which became a full-blown pandemic after 12 mutations. (Related: Cholera Epidemic - Homeopathy holds healing and empowerment for the Haitian people.)
To counter the devastating effects of this disease, as well as other harmful bacteria, researchers have developed drugs that will serve as the last line of defense against acute infection. Cationic antimicrobial peptides, or CAMPs, are an example of last-line drugs. The peptides bind themselves to a bacterium's cell wall, allowing them to "break in" and, ultimately, destroy it. While this has been effective for many bacterial infections, the current pandemic biotype is known to be resistant to these peptides, as compared to previous pandemic strains. An enzyme found in the bacterium is able to remodel its cell wall to match the peptides. This gives the bacteria resistance to the effects of CAMPs.
The research team's previous papers had already detailed two of the proteins that make are part of the modification process.
In this paper, lead author Jeremy Henderson explained how the enzyme AlmG was able to disguise the cell wall. This process was done when AlmG connects glycine, the smallest and simplest of amino acids, to lipid A, one of the materials that make up the outer membrane of a bacterial cell. This process changes the charge of lipid A molecules, which keeps CAMPs from binding.
The study pointed out that while modifying Lipid A is a defense mechanism that is noted in other bacteria, the manner by which cholera was able to modify this process is unique.
"It became apparent over the course of our work that how [this enzyme] improves shield functionality is quite different than would be expected based on what we know about groups of enzymes that look similar," Henderson explained. "It just opens up the door for this operating with a completely different mechanism than what's been described in the literature for related proteins."
Still, researchers warn that the antibiotic resistance posed by V. cholerae is a "potential concern" if it affects bacteria that are already drug-resistant.
Sources include:
Please contact us for more information.